The popular press (including high profile outlets like the New York Times) recently covered an article from the New England Journal of Medicine (NEJM) regarding three women blinded by what was described as “stem cell therapy” for macular degeneration, all by the same center. Harriet Hall ably covered this in a previous SBM post, but there is some interesting background information that I do not think has been covered elsewhere and is worth discussing. Most notably, it turns out there was a body of literature that predicted the tragic outcomes, but did not seem to have informed patient care.
The putative stem cells were introduced in the eye by intravitreal injection. A previous SBM article provides some background on macular degeneration, and also described how science-based treatment with drugs delivered via intravitreal injection, has revolutionized the treatment of exudative macular degeneration.
The NEJM article never specifically identifies the stem cell clinic that treated the three patients however, they do report a registry number from ClinicalTrials.gov that links to a company called Bioheart in Sunrise, Florida. Bioheart is now known as U.S. Stem Cell. In 2013 Bioheart announced their intent to perform a clinical trial using stem cells derived from adipose (fat) tissue to treat macular degeneration. They created an entry on the clinical trial registry website ClinicalTrials.gov.
Clinical trials registries were created to improve transparency in the conduct and reporting of clinical trials. Registries are publicly accessible and searchable, giving patients information about clinical trials relevant to their condition.
Two of the patients were introduced to Bioheart via ClinicalTrials.gov. According to the NEJM article, at least one of the patients believed they were participating in a clinical trial, however:
- It does not appear that Bioheart or U.S. Stem Cell ever enrolled any patients into a research trial for macular degeneration
- The patients described in the NEJM article deviated from the ClinicalTrials.gov protocol in crucial ways
- The ClinicalTrials.gov entry clearly states that the study was withdrawn with zero patients enrolled
I could find no entries for any other macular degeneration trials registered to Bioheart or U.S. Stem Cell.
The stories of the three patients
All three patients were seeking treatment for age-related macular degeneration and all had suffered some degree of vision loss. Despite the diagnosis, all had reasonably good vision in at least one eye; sufficient to qualify for a driver’s license in their respective home-states. All three paid $5,000 for the privilege of having injections of putative stem cells into both eyes. All three ended up legally blind shortly after treatment.
The procedures involved harvesting stem cells from the patients’ own periumbilical fat (fat in the lower belly) using liposuction. The liposuction material was washed, the cellular component treated with an enzyme solution, centrifuged, washed, and centrifuged again. The cells were suspended in platelet-rich plasma (taken from the patients’ own blood) then injected into both eyes.
Things went downhill quickly after that. All patients suffered decreased vision and sought treatment at academic medical centers from two days to one week after treatment. Among the more common findings were retinal detachment, development of retinal scar tissue, bleeding in the retina and in the vitreous, and dislocated lenses. Despite appropriate interventions, including surgery, all three patients ended up legally blind. One patient lost all perception of light in both eyes. One patient lost all but the ability to see light in one eye, and could see only movement in the other. The patient with the best outcome had 20/200 vision in her better-seeing eye, qualifying her as “legally blind.”
According the NEJM article, the patients had signed a consent form informing them of the risk of blindness, which is pretty standard for any invasive ocular procedure. However, as every ethicist and lawyer will tell you, informed consent is more than having the patient sign a form. How can one truly inform a patient about the risk-to-benefit ratio of intravitreal injection of adipose-derived tissue for macular degeneration? There are no credible sources of information to estimate the benefit. The folks at Bioheart expressed intent to do a clinical trial; that would have provided some basis for providing risk and benefit estimates, but it appears that they decided to skip that step and go directly to the fee-for-service market.
When it comes to risk, however, there was good reason to anticipate complications.
Is stem cell treatment good for macular degeneration?
It is too soon to be absolutely certain, but in my opinion injection of adipose-derived cells into the vitreous, as performed by Bioheart, is unlikely to ever be an effective treatment for macular degeneration.
In order for a stem cell therapy to be effective, several things have to happen and several things have to not happen. The stem cells have to get to the tissue in need of repair or replacement. They have to differentiate into the appropriate type of cell needed to repair or replace the malfunctioning tissue. They have to integrate into the tissues in a way that they improve the function of the organ in which they are needed. None of these are trivial steps, especially in the retina, which is essentially part of the brain. I find no animal models or human studies suggesting that the strategy practiced by Bioheart is likely to work.
The usual sequence in developing a new treatment is to first determine if a treatment is safe: primum non noceri. This usually involves a step-wise sequence of preclinical studies followed by carefully-monitored clinical studies. It is important to consider not just the active ingredients, but everything in the preparation. It appears that Bioheart skipped these steps entirely.
It turns out that some of this work had already been done. Years ago researchers had studied the effects of the intravitreal injection of one of the components of Bioheart’s “stem cell” treatment. The results were not encouraging, and foreshadowed the complications experienced by the patients treated by Bioheart.
What went wrong?
To say that these patients received stem cell treatment is an oversimplification. They received a biologic soup composed of whatever cells were in the liposuction and platelet rich plasma.
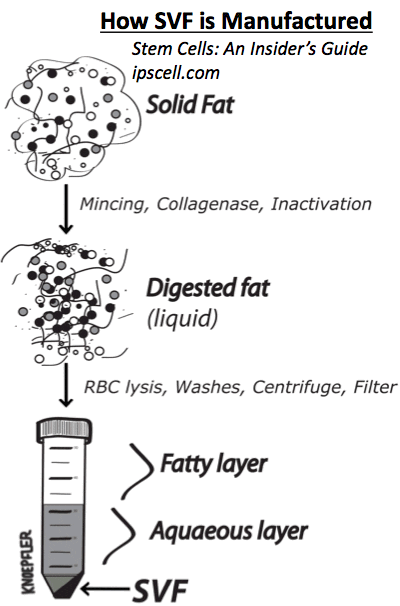
Stromal vascular fraction, an extract of cleaned, centrifuged stem cells derived from body fat (as performed properly by a responsible lab).
There are many ways such a strategy could go wrong, and a very limited number of scenarios in which things could go right. The eye is very unforgiving when it comes to the wrong types of cells proliferating in the wrong place, or exposure to chemicals which may be uniquely toxic to structures in the eye.
What the folks at Bioheart did not know (or knew and disregarded?) is that platelet-rich plasma (one of the ingredients in the stem cell soup) has been used in animal models, including multiple species, to create complex retinal detachments resembling those that their patients developed. What was done in these patients mimicked animal models known to stimulate scar tissue formation and retinal detachments. There is substantial published literature on this subject. It took me just a couple of minutes to find this article and several others by simply performing a Google search with terms “platelet-rich plasma” and “vitreous.”
Injection of platelet-rich plasma into the vitreous was likely a provocative factor in some of the complications suffered by these three unfortunate patients. It is also highly plausible that stem cells or other more mature cell types from the liposuction potentiated the scar tissue formation.
Another common finding in the three patients was lens dislocation. This is a quite unusual and puzzling outcome. Authors of the NEJM article speculated that the enzymes used in the preparation of the stem cells may have remained in the final injection, causing weakening of the fibers (zonules) that normally hold the lens in position. This is a plausible hypothesis and raises the possibility that other reagents used in the preparation of the “stem cells” may have contaminated the injection and contributed to the toxicity.
Two lawsuits have been filed against U.S. Stem Cell for alleged blindness due to stem cell treatment. Paul Knoepfler, a stem cell researcher, vocal advocate of responsible use of stem cells, and contributor to SBM, has blogged about this. The allegations against U.S. Stem Cell and the providers working with them, if true, are quite disturbing.
A recent news article ties together the legal actions with some of the other facts surrounding these cases. Three people are named as significant players in this drama. Kristin Comella has been the Chief Scientific Officer of U.S. Stem Cell (and of its prior identity, Bioheart) since 2010. She is featured prominently on their website.
According to her bio she is a “world renowned expert on regenerative medicine with a focus on adipose derived stem cells.” Her education includes a Bachelor’s and Master’s Degree in chemical engineering and she ” is a PhD candidate in Biomedical Engineering at Florida International University” (emphasis mine).
Alejandro Perez is a Nurse Practioner. His bio states that he graduated from the University of Havana as a Doctor in Medicine, but makes no claim that he is licensed to practice medicine in the U.S. It is alleged that Mr. Perez was introduced as a physician and performed intravitreal injections.
Lacking in-house expertise in ophthalmology, a local private-practice ophthalmologist, Shareen Greenbaum, MD was engaged to collaborate with Bioheart. By her own admission Dr. Greenbaum had little or no experience in medical research or clinical research. A 2013 press release announced she would be Principal investigator for a clinical trial of adipose-derived stem cells for macular degeneration. The clinical trial was never initiated, however Dr. Greenbaum continued to participate in the treatment of patients outside the constraints of a clinical trial.
If Bioheart had engaged someone with genuine expertise in ocular physiology, ocular cell biology, or someone with experience in translational ocular research, it is likely that they would have had the insight to put the brakes on what turned out to be a treatment with catastrophic complications.
Parting observations
The clinical trial that never was
Biohart announced a clinical trial and registered in ClinicalTrials.gov, yet it appears that they skipped this step and went directly to treating patients with macular degeneration. The ClinicalTrials.gov entry drew a couple of the patients to Bioheart. An entry on ClinicalTrials.gov is sometimes inferred by some patients as demonstrating oversight by the FDA, however it is only a registry and requires no review or approval by the government. At least one of the patients thought she was enrolled in a clinical trial.
Had the three women been treated according to the standards described for the Bioheart clinical trial registered at ClinicalTrials.gov they probably would have fared better than they did. For the most part, their vision was too good to be eligible for the clinical trial. The study protocol also dictated that only one eye should be treated.
A clinical trial requires oversight by in Institutional Review Board (IRB): an organization charged with protecting the interests of research subjects. A responsible IRB might have asked appropriate questions and insisted on appropriate safeguards to ensure that this treatment was undertaken with appropriate caution. However, IRB oversight is no guarantee of the legitimacy of a treatment. Some stem cell centers are notorious for “pay to play” clinical trials with little or no scientific rigor.
Double trouble!
The providers opted to treat both eyes of these patients with the same treatment on the same day; a dubious decision for a treatment without an established track-record of safety. Had they simply opted to treat one eye, the tragedy suffered by these patients would have been much less.
No adult supervision
Review of U.S. Stem Cell’s (formerly Bioheart’s) corporate leadership, and scientific advisory board shows no particular expertise in diseases, treatment, or research on the eye. When they announced the intent to conduct a clinical trial on stem cell treatment for macular degeneration, the Principal Investigator was an ophthalmologist with little experience in basic or clinical research.
U.S. Stem Cell describes using adipose-derived stem cells to treat a variety of conditions. It is naïve to believe that a treatment that can safely be injected into joints or muscles will be equally well tolerated in the eye. The eye can be very unforgiving in respect the wrong types of cells growing in the wrong place at the wrong time. Had Bioheart employed or consulted with someone with a working knowledge of ocular physiology, ocular cell biology, or translational research in ocular therapies, it is likely they would have understood the hazard of injecting a slurry composed of adipose-derived cells and blood products into the eye.
Even in the absence of expert personnel, there are powerful tools freely available to review the existing literature on just about any subject. Even a cursory search should have disclosed a body of research that foreshadowed the tragic complications suffered by the patients they treated.
